Ranitidine Ban: Reasons, Risks, and Safer Alternatives Explained

Ranitidine Ban was enforced worldwide due to concerns over NDMA contamination, a probable carcinogen. Once widely used to treat acid reflux, ulcers, and GERD, ranitidine faced scrutiny after studies detected unsafe levels of NDMA in its formulation. Health authorities, including the FDA and EMA, recalled the drug, urging patients to switch to safer alternatives like famotidine and proton pump inhibitors (PPIs). The ban raised concerns about long-term health risks and the safety of acid-reducing medications. Understanding the reasons behind the ban helps individuals make informed decisions about their treatment options and protect their health.
What is Ranitidine?
Ranitidine, a histamine-2 (H2) receptor antagonist, actively reduces stomach acid production. Doctors commonly prescribed it to treat conditions like acid reflux, ulcers, and Zollinger-Ellison syndrome.
Forms of Ranitidine
Ranitidine came in multiple forms, including:
- Tablets (75 mg, 150 mg, 300 mg)
- Syrup (Liquid oral formulation)
- Injectable Solution (Used in hospitals for severe cases)
Route of Administration
Doctors administered ranitidine in several ways:
- Oral Route: Patients took tablets or syrup by mouth.
- Intravenous (IV) Injection: Hospitals used this method for immediate relief.
- Intramuscular (IM) Injection: Though less common, it remained available for specific cases.
Mechanism of Action
Ranitidine blocks H2 receptors in the stomach lining, preventing gastric acid release. By reducing acid, it alleviates symptoms of heartburn, GERD, and ulcers.
When Doctors Used Ranitidine
Doctors prescribed ranitidine for treating:
- Gastroesophageal Reflux Disease (GERD): It effectively reduced acid reflux symptoms.
- Peptic Ulcers: The drug promoted ulcer healing in the stomach and intestines.
- Zollinger-Ellison Syndrome: It controlled excessive stomach acid production.
- Heartburn and Indigestion: Patients used it for short-term relief of acid-related discomfort.
Adverse Effects of Ranitidine

Although many tolerated ranitidine well, some experienced side effects:
- Common Side Effects:
- Headache
- Nausea
- Diarrhea or constipation
- Dizziness
- Severe Side Effects:
- Liver dysfunction
- Blood disorders (rare)
- Allergic reactions (rash, swelling, difficulty breathing)
- Carcinogenic Concerns: The discovery of N-nitrosodimethylamine (NDMA), a potential carcinogen, raised safety alarms and led to the ban.
Price of Ranitidine
Before the ban, ranitidine remained an affordable option:
- United States: $5–$15 per pack (generic)
- Bangladesh: BDT 100–500 per strip
- India: ₹50–₹200 per pack Prices varied based on brand and dosage.
Which Countries Banned Ranitidine and Why?
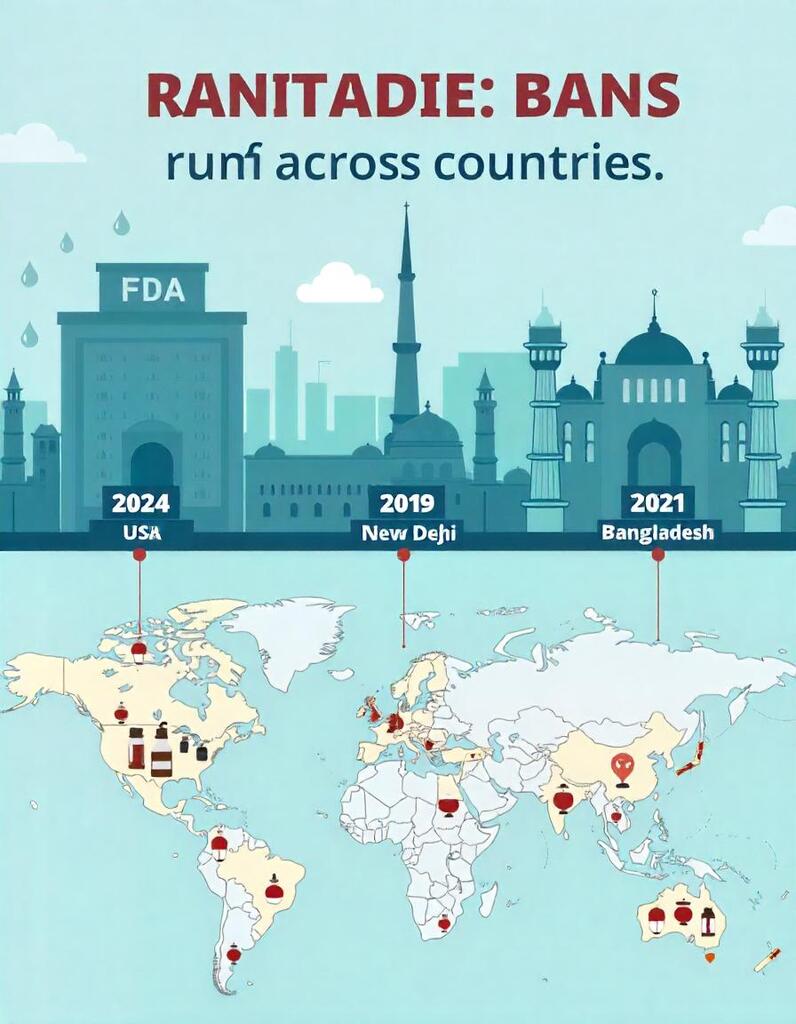
Health authorities banned ranitidine due to NDMA contamination, a probable human carcinogen. Here’s a timeline of major bans:
- United States (April 2020) – The FDA withdrew all ranitidine products.
- Europe (April 2020) – The European Medicines Agency (EMA) recommended a recall.
- India (2020) – The Drug Controller General of India (DCGI) suspended sales.
- Bangladesh (2020) – The Directorate General of Drug Administration (DGDA) banned ranitidine-based medicines.
- Canada, Australia, and other countries also recalled ranitidine.
Alternative Drugs to Ranitidine
Safer alternatives include:
- H2 Receptor Antagonists:
- Famotidine (Preferred alternative with lower cancer risk)
- Cimetidine
- Proton Pump Inhibitors (PPIs):
- Omeprazole
- Esomeprazole
- Pantoprazole These medications effectively reduce acid production and remain widely prescribed.
Frequently Asked Questions (FAQs) about Ranitidine Ban
1. Why did authorities ban ranitidine?
Regulators found NDMA contamination, which increases cancer risk.
2. Is ranitidine still safe to use?
No, major health agencies advise against it.
3. What works best as a substitute for ranitidine?
Doctors recommend famotidine or PPIs like omeprazole.
4. Can people still find ranitidine in some countries?
Some regions may have it, but most countries pulled it from the market.
5. How does famotidine compare to ranitidine?
Famotidine works similarly but carries a lower cancer risk.
Conclusion about Ranitidine Ban
Ranitidine was once a go-to medication for acid-related disorders. However, its link to NDMA contamination resulted in a global ban. Today, safer options like famotidine and PPIs replace it. If you previously used ranitidine, consult a healthcare provider to explore better alternatives.
For more information, visit the FDA website.
